Phase 3 COVE Study: Updated cases show continued strong efficacy, including greater than 90% against cases of COVID-19 and greater than 95% against severe cases of COVID-19, with approximately 6 months median follow-up post dose 2
Antibody persistence data out to 6 months published in The New England Journal of Medicine
New preclinical data shows variant-specific booster vaccine candidates (mRNA-1273.351 and mRNA-1273.211) increase neutralizing titers against SARS-CoV-2 variants of concern
Supply Update: As of April 12, approximately 132 million doses have been delivered globally
Vaccines Day to be held on Wednesday, April 14 at 8:00 a.m. ET
Moderna, Inc. (Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced clinical and supply updates on its COVID-19 Vaccine program. New results from a preclinical study of the Company’s COVID-19 variant-specific vaccine candidates showed that the Company’s variant-specific booster vaccine candidates (mRNA-1273.351 and mRNA-1273.211) increase neutralizing titers against SARS-CoV-2 variants of concern. To date, the Company has delivered approximately 132 million doses of the Moderna COVID-19 Vaccine globally.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210413006131/en/
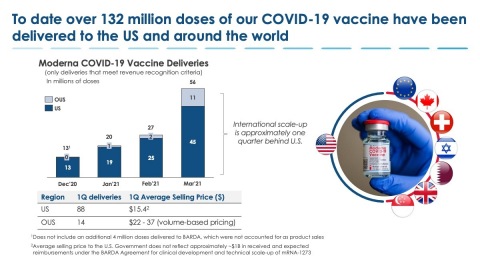
To date, over 132 million doses of Moderna's COVID-19 vaccine have been delivered to the U.S. and around the world. (Graphic: Business Wire)
“The Moderna team continues to make important progress with our COVID-19 Vaccine. We are looking forward to having the clinical data from our variant-specific booster candidates, as well as clinical data from the Phase 2/3 study of our COVID-19 Vaccine in adolescents,” said Stéphane Bancel, Chief Executive Officer of Moderna. “The new preclinical data on our variant-specific vaccine candidates give us confidence that we can proactively address emerging variants. Moderna will make as many updates to our COVID-19 vaccine as necessary until the pandemic is under control.”
Update on the Phase 3 COVE Study of the Moderna COVID-19 Vaccine
Today, the Company is sharing an update on the Phase 3 COVE study of the Moderna COVID-19 Vaccine (mRNA-1273). An updated review of adjudicated cases has identified over 900 cases of COVID-19 in the COVE study as of April 9th, including over 100 cases of severe COVID-19, as defined in the protocol, with a median follow-up of approximately 6 months post dose 2. Vaccine efficacy starting two weeks following the second dose and based on the updated adjudicated cases remains consistent with prior updates, including greater than 90% against all cases of COVID-19, and greater than 95% against severe cases of COVID-19.
As of April 13, all placebo participants have been offered the Moderna COVID-19 Vaccine and 98% of those have received the vaccine.
The COVE study is ongoing and reported results remain preliminary. Throughout the year, Moderna will be sharing updated data from the Phase 3 COVE study including efficacy against asymptomatic infection, genotyping data, additional antibody persistence data and information regarding potential correlates of protection.
The Phase 2/3 TeenCOVE study of mRNA-1273 at the 100 µg dose level in adolescents ages 12-17 is fully enrolled with approximately 3,000 participants in the U.S.
The Phase 2/3 KidCOVE study of mRNA-1273 in the pediatric population ages 6 months-11 years is currently enrolling. The Company expects to enroll 6,750 healthy pediatric participants in the U.S. and Canada into this two-part, dose escalation study. In Part 1, each participant ages 2 years to less than 12 years may receive one of two dose levels (50 μg or 100 μg). Also in Part 1, each participant ages six months to less than 2 years may receive one of three dose levels (25 μg, 50 μg and 100 μg). An interim analysis will be conducted to determine which dose will be used in Part 2, the placebo-controlled expansion portion of the study.
Antibody Persistence Data out to 6 Months Published in The New England Journal of Medicine
Antibody persistence data out to 6 months following the second dose of the Moderna COVID-19 Vaccine were recently published in The New England Journal of Medicine. This study analyzed 33 healthy adult participants in the NIH-led Phase 1 study of Moderna’s COVID-19 Vaccine at 6 months following the second 100 μg dose (day 209). As detected by three distinct serologic assays, antibodies elicited by the Moderna COVID-19 Vaccine persisted through 6 months after the second dose. Antibody decay was estimated using two approaches and was consistent with published observations of convalescent patients with COVID-19 through 8 months after symptom onset.
Preclinical Data on Variant-Specific Booster Candidates
New preclinical data on the Company’s variant-specific booster vaccine candidates have been submitted as a preprint to bioRxiv and will be submitted for peer-reviewed publication. These variant-specific vaccine candidates include mRNA-1273.351, which is more specifically targeted against the SARS-CoV-2 variant known as B.1.351 first identified in the Republic of South Africa, and a multivalent booster candidate, mRNA-1273.211, which combines mRNA-1273 (Moderna’s authorized vaccine against ancestral strains) and mRNA-1273.351 in a single vaccine.
Both mRNA-1273.351 and mRNA-1273.211 increase neutralizing titers against SARS-CoV-2 variants of concern in Balb/c mice. Specifically, this preclinical data confirms improved neutralizing titers with the mRNA-1273.351 vaccine primary series. The multi-valent vaccine provided the broadest level of immunity. A boost at 6 months with mRNA-1273.351 closed the neutralizing titer gap for the variants of concern. Following the mRNA-1273.351 boost, neutralizing titers were comparable between the ancestral strain (Wuhan) and the new B.1.351 variant. The Company’s Phase 2 study to evaluate three approaches to boosting is ongoing.
Global Supply Update
In the fourth quarter of 2020, the Company delivered approximately 17 million doses to the U.S. government. For the first quarter of 2021, the Company delivered approximately 88 million doses to the U.S. government and approximately 14 million doses to other customers. Through April 12, 2021, Moderna cumulatively delivered approximately 132 million doses globally, including approximately 117 million doses to the U.S. government and approximately 15 million doses delivered from Moderna’s ex-U.S. supply chain. The Company remains on track to deliver the second 100 million doses to the U.S. government by end of May 2021 followed by another 100 million additional doses by end of July 2021. The ex-U.S. supply chain was established approximately one quarter behind the U.S. supply chain and continues to ramp up.
Cerebral Venous Sinus Thrombosis (CVST) or Thrombotic Events
On April 13, the Company issued a statement on cerebral venous sinus thrombosis (CVST). A comprehensive assessment of the totality of the available safety data for mRNA-1273 after over 64.5 million doses administered globally does not suggest an association with CVST or thrombotic events, based on analyses performed using data through March 22, 2021. The number of vaccinations was derived from the U.S. Centers for Disease Control and Prevention (CDC) website, European Centre for Disease Prevention and Control (ECDC) website and inferred for other countries based on distribution and the proportion of doses distributed administered in ex-U.S. settings.
Vaccines Day Tomorrow
Moderna is hosting a Vaccines Day tomorrow, Wednesday April 14th, beginning at 8:00 a.m. ET. A live webcast will be available under “Events and Presentations” in the Investors section of the Moderna website at investors.modernatx.com. A replay of the webcast will be archived on Moderna’s website for one year following the presentation.
About the Moderna COVID-19 Vaccine
The Moderna COVID-19 Vaccine is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike (S) protein, which was co-developed by Moderna and investigators from the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center. The first clinical batch, which was funded by the Coalition for Epidemic Preparedness Innovations, was completed on February 7, 2020 and underwent analytical testing; it was shipped to the National Institutes of Health (NIH) on February 24, 2020, 42 days from sequence selection. The first participant in the NIAID-led Phase 1 study of the Moderna COVID-19 Vaccine was dosed on March 16, 2020, 63 days from sequence selection to Phase 1 study dosing. On May 12, 2020, the U.S. FDA granted the Moderna COVID-19 Vaccine Fast Track designation. On May 29, 2020, the first participants in each age cohort were dosed in the Phase 2 study of the vaccine. On July 8, 2020, the Phase 2 study completed enrollment.
Results from the second interim analysis of the NIH-led Phase 1 study of the Moderna COVID-19 Vaccine in the 56-70 and 71+ age groups were published on September 29, 2020 in The New England Journal of Medicine. On November 30, 2020, Moderna announced the primary efficacy analysis of the Phase 3 study of the vaccine conducted on 196 cases. On November 30, 2020, the Company also announced that it filed for Emergency Use Authorization with the U.S. FDA and a Conditional Marketing Authorization (CMA) application with the European Medicines Agency. On December 18, 2020, the U.S. FDA authorized the emergency use of the Moderna COVID-19 Vaccine in individuals 18 years of age or older. Moderna has also received authorization for its COVID-19 vaccine from health agencies in Canada, Israel, the European Union, the United Kingdom, Switzerland, Singapore, Qatar and Taiwan. Additional authorizations are currently under review in other countries and by the World Health Organization.
The Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS) is supporting the continued research and development of the Company’s COVID-19 vaccine development efforts with federal funding under contract no. 75A50120C00034. BARDA is reimbursing Moderna for 100 percent of the allowable costs incurred by the Company for conducting the program described in the BARDA contract. The U.S. government has agreed to purchase supply of mRNA-1273 under U.S. Department of Defense contract no. W911QY-20-C-0100.
AUTHORIZED USE
Moderna COVID-19 Vaccine is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older.
IMPORTANT SAFETY INFORMATION
- Do not administer the Moderna COVID-19 Vaccine to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of the Moderna COVID-19 Vaccine.
- Appropriate medical treatment to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the Moderna COVID-19 Vaccine. Monitor Moderna COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
- Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished response to the Moderna COVID-19 Vaccine.
- The Moderna COVID-19 Vaccine may not protect all vaccine recipients.
- Adverse reactions reported in a clinical trial following administration of the Moderna COVID-19 Vaccine include pain at the injection site, fatigue, headache, myalgia, arthralgia, chills, nausea/vomiting, axillary swelling/tenderness, fever, swelling at the injection site, and erythema at the injection site.
- Severe allergic reactions, including anaphylaxis, have been reported following administration of the Moderna COVID-19 Vaccine during mass vaccination outside of clinical trials.
- Available data on Moderna COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy. Data are not available to assess the effects of Moderna COVID-19 Vaccine on the breastfed infant or on milk production/excretion.
- There are no data available on the interchangeability of the Moderna COVID-19 Vaccine with other COVID-19 vaccines to complete the vaccination series. Individuals who have received one dose of Moderna COVID-19 Vaccine should receive a second dose of Moderna COVID-19 Vaccine to complete the vaccination series.
- Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Moderna COVID-19 Vaccine.
- Vaccination providers must complete and submit reports to VAERS online at https://vaers.hhs.gov/reportevent.html. For further assistance with reporting to VAERS, call 1-800-822-7967. The reports should include the words “Moderna COVID- 19 Vaccine EUA” in the description section of the report.
Click for Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) and Full EUA Prescribing Information for more information.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: the Company’s development of a vaccine (mRNA-1273) to protect against the SARS-CoV-2 virus, which causes COVID-19; the ability of the Moderna COVID-19 Vaccine to protect against COVID-19 over time; the Company’s ability to develop vaccines against variants of the SARS-CoV-2 virus and the protection conferred by those variant-specific vaccines; the Company’s sharing of additional information about the Moderna COVID-19 Vaccine and the Phase 3 COVE Study; plans for adolescent and pediatric clinical studies of the Moderna COVID-19 Vaccine and its potential administration in adolescent and pediatric populations; the need for booster vaccines against the SARS-CoV-2 virus and its variants; the duration of protection against SARS-CoV-2 from existing vaccines; the Company’s supply of its COVID-19 vaccine to the U.S. government and other customers and the anticipated timing for those deliveries; and the lack of association between administration of mRNA-1273 and CVST or thrombotic events. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “could,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others: the fact that there has never been a commercial product utilizing mRNA technology approved for use; the fact that the rapid response technology in use by Moderna is still being developed and implemented; the safety, tolerability and efficacy profile of the Moderna COVID-19 Vaccine observed to date may change adversely in ongoing analyses of trial data or subsequent to commercialization; the Moderna COVID-19 Vaccine may prove less effective against variants of the SARS-CoV-2 virus, or the Company may be unsuccessful in developing future versions of its vaccine against these variants; despite having ongoing interactions with the FDA or other regulatory agencies, the FDA or such other regulatory agencies may not agree with the Company’s regulatory approval strategies, components of our filings, such as clinical trial designs, conduct and methodologies, or the sufficiency of data submitted; Moderna may encounter delays in meeting manufacturing or supply timelines or disruptions in its distribution plans for the Moderna COVID-19 Vaccine; whether and when any biologics license applications and/or additional emergency use authorization applications may be filed in various jurisdictions and ultimately approved by regulatory authorities; potential adverse impacts due to the global COVID-19 pandemic such as delays in regulatory review, manufacturing and clinical trials, supply chain interruptions, adverse effects on healthcare systems and disruption of the global economy; and those other risks and uncertainties described under the heading “Risk Factors” in Moderna’s most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC) and in subsequent filings made by Moderna with the SEC, which are available on the SEC’s website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna’s current expectations and speak only as of the date hereof.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210413006131/en/
Contacts
Media:
Colleen Hussey
Director, Corporate Communications
617-335-1374
Colleen.Hussey@modernatx.com
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
617-209-5834
Lavina.Talukdar@modernatx.com













