Repeat doses of neffy under nasal allergen challenge demonstrate a pharmacokinetic profile greater than or similar to injection, and a pharmacodynamic profile greater than injection
Company believes completion of repeat dosing study and nitrosamine assessments address the deficiencies identified in the U.S. Food and Drug Administration (FDA)’s Complete Response Letter (CRL)
NDA on track to respond to CRL early in the second quarter of 2024 followed by an expected six-month review period
SAN DIEGO, Feb. 20, 2024 (GLOBE NEWSWIRE) -- ARS Pharmaceuticals, Inc. (NASDAQ: SPRY), a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect patients from severe allergic reactions that could lead to anaphylaxis, today announced topline results from its clinical study comparing repeat doses of neffy (epinephrine nasal spray) to repeat doses of epinephrine intramuscular (IM) injection, as requested by the FDA with and without nasal allergen challenge (NAC) conditions.
“We are very pleased with the topline results from our repeat dose study of neffy under nasal allergen challenge conditions, which we believe will address FDA’s requests in their Complete Response Letter and support the approval of neffy for the treatment of Type I allergies, including anaphylaxis,” said Richard Lowenthal, Co-Founder, President and CEO of ARS Pharma. “The study objective was to compare twice dosing with epinephrine injection and twice dosing with neffy under normal conditions and after nasal allergen challenge. The results show that the neffy pharmacokinetic and pharmacodynamic profile is greater than or similar to intramuscular injection, the comparator FDA requested for this study. In particular, repeat dosing in the same nostril was greater in exposure than dosing once in each nostril and greater than injection on both PK exposures and PD response at all time points, which may help inform labeling and instructions for use. With these results, we are completing the necessary work to submit our response to FDA in the next couple of months. We look forward to working with the Agency in our efforts to make neffy available to patients as soon as possible.”
Based on the company’s analysis of the data, we believe these results should support filing our response to the FDA’s CRL. The repeat dose study under NAC conditions was designed with the FDA to address the Agency’s outstanding questions regarding neffy as described in the Complete Response Letter (CRL) from September 2023. FDA did not provide guidance on a prespecified set of endpoints, but the data are anticipated to be informative to labeling if a second dose of neffy is needed.
ARS Pharma also completed the nitrosamine testing requested in the CRL per FDA’s draft guidance issued in August 2023, with no measurable levels of nitrosamines detected.
Previously reported data with a single dose of neffy under NAC conditions showed accelerated absorption of neffy with exposures higher than IM injection during the early time period when clinical response to epinephrine is expected.
“When a severe, life-threatening allergic reaction occurs, it is necessary to administer epinephrine as soon as possible. Based on the single dose NAC data, my allergy colleagues and myself already believed that neffy would be effective under NAC conditions. FDA asked what would happen if NAC conditions occurred during the 10% of anaphylaxis events that require a repeat dose of epinephrine,” says Thomas B. Casale, M.D., Professor of Medicine and Pediatrics and Chief of Translational Research in Allergy/Immunology at the University of South Florida in Tampa. “This study answers this question - exposures with repeat doses of neffy under NAC are at least as good as repeat doses of injection, and dosing in the same nostril is greater than injection. I believe the robust pharmacodynamic effect observed with repeat dosing in the same nostril or opposite nostrils compared to injection during nasal allergen challenge shows that neffy will be at least as effective as injection in reversing anaphylaxis symptoms, even during infrequently observed rhinitis of note. Together with the 100% response rate recently reported for neffy in treating pediatric oral food challenge-induced anaphylaxis, I believe this repeat dose NAC data provides further clinical evidence that neffy is effective under all relevant clinical conditions, and, if approved, supports its potential to provide an effective needle-free option for severe allergy patients and their families.”
ARS Pharma intends to submit this repeat dose NAC study data to the FDA as part of its response to the CRL early in the second quarter of 2024.
“The safety and health of severe allergy patients is our shared priority. The neffy repeat dose NAC study should address the theoretical question FDA identified in the small subset of allergy patients who experience rhinitis and may require a second dose of epinephrine,” says Sung Poblete, PhD, RN, CEO of FARE. “We are gratified that these data help to provide a complete picture of neffy use in the real world, and hopefully will resolve FDA’s stated residual uncertainty. Now, nearly a year after the FDA Advisory Committee voted for its approval without any additional studies, we urge the FDA to move quickly to approve neffy and provide our community with a long-awaited, needle-free epinephrine treatment option. Allergy patients and their families are waiting.”
neffy Repeat Dosing Study Design
The randomized, crossover pharmacokinetic (PK) and pharmacodynamic (PD) study enrolled 43 patients with seasonal allergic rhinitis who tested positive with a Total Nasal Symptom Score (TNSS) of ≥5 out of 12 and a congestion score of ≥2 out of 3 during the screening NAC. NAC involves spraying purified antigen directly onto the nasal mucosa – a ‘worst-case’ experimental condition in contrast to real-world nasal conditions such as upper respiratory tract infections or acute allergic rhinitis from natural causes. All patients were dosed with epinephrine within 15 minutes of induction of the NAC during the peak effect, without allowing time for nasal symptoms to subside. A second dose of epinephrine was given to patients 10 minutes after the first dose per FDA labeling of epinephrine products.
FDA explicitly requested that ARS Pharma include intramuscular (IM) epinephrine given via manual syringe as the comparator in this study, as IM is the reference-listed drug and historical basis for efficacy of all other epinephrine products. Meta-analyses (Patel et al, JACI 2021) analyzing over 30,000 anaphylaxis events demonstrate that about 90% of events respond to a single dose of epinephrine injection irrespective of the delivery device, despite significant differences in PK.
PK/PD Study Results
Data from the company’s primary analysis of the completed study showed that responses on pharmacodynamic (PD) surrogate markers for efficacy in anaphylaxis, such as systolic blood pressure and heart rate, correlated well with pharmacokinetic (PK) exposures and were consistently higher for repeat doses of neffy, irrespective of dosing in the same nostril (R/R) or opposite nostrils (R/L), compared to repeat doses of IM injection. Dosing in the same nostril (R/R) resulted in higher PD than injection at all time points measured, while dosing in the opposite nostril (R/L) was higher than injection until the 40 to 60 minute time points, after which PD was indistinguishable from injection.
Consistent with prior studies, significant responses on these PD surrogate markers of efficacy with neffy were observed even at one minute after dosing. The PD responses demonstrate that the epinephrine exposures achieved with repeat doses of neffy (R/R or R/L) fully activate the receptors involved in reversing anaphylaxis symptoms.
Figure 1: Mean Change from Baseline in Systolic Blood Pressure (mm Hg)
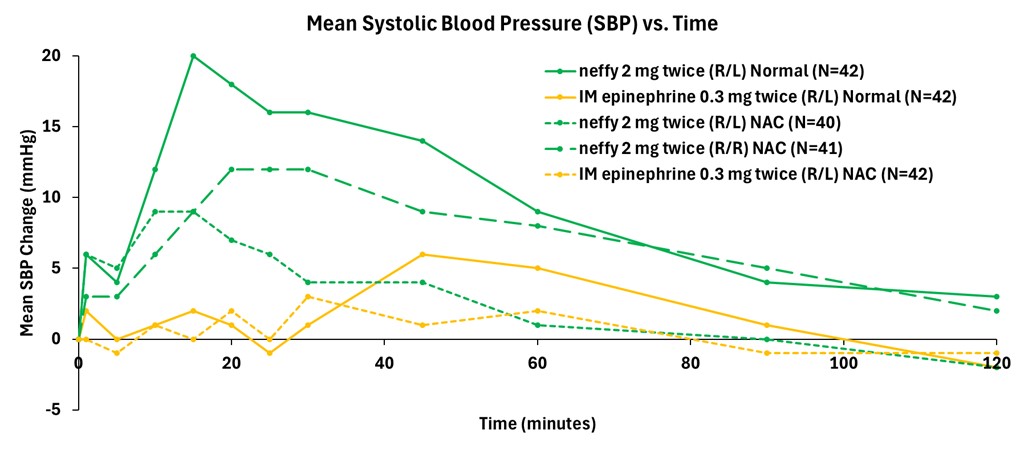
Figure 2: Mean Change from Baseline in Heart Rate (bpm)
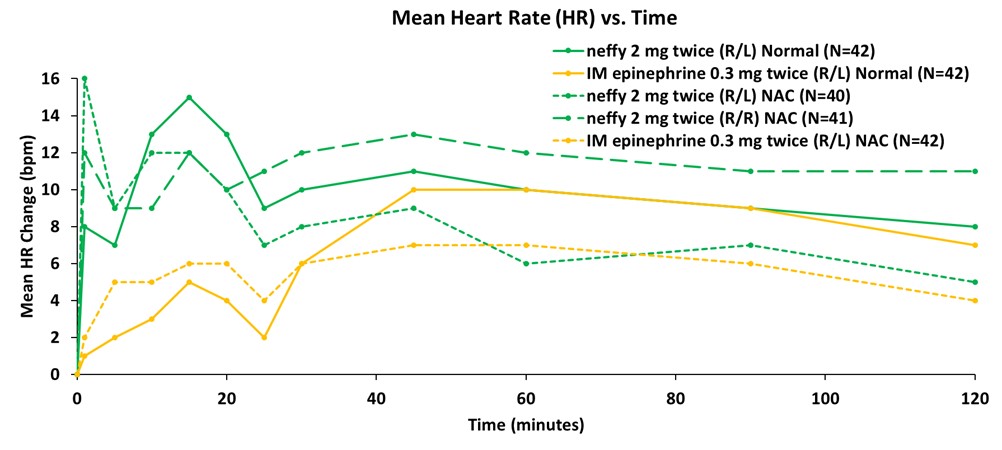
Figure 3: Mean Epinephrine Plasma Concentrations (pg/mL)
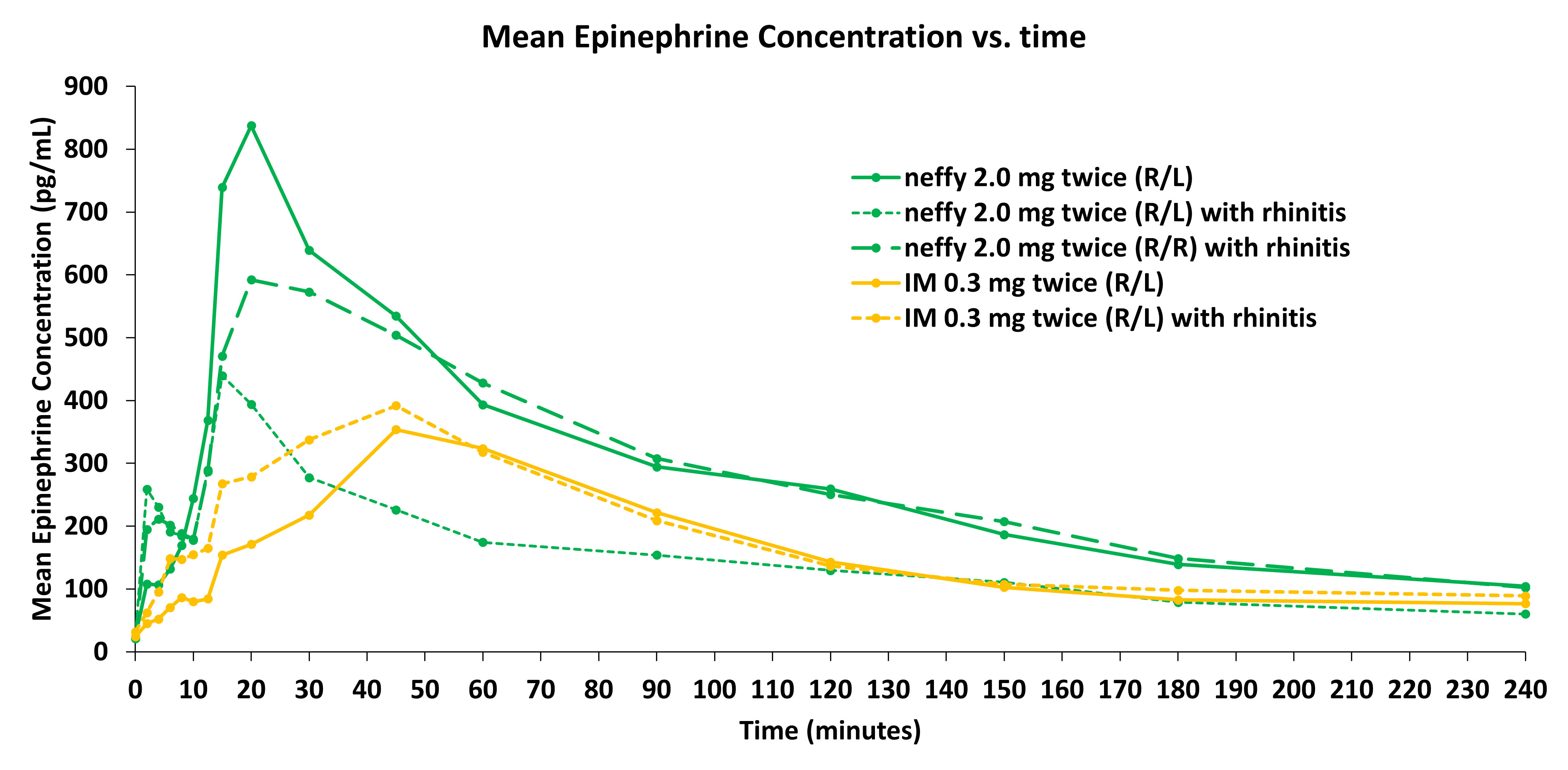
The clinical data also demonstrated that mean epinephrine concentrations following repeat doses of 2 mg neffy in the same nostril (R/R) were numerically higher than repeat doses of 0.3 mg injection at all time points through at least 240 minutes under NAC conditions.
Mean epinephrine concentrations of 2 mg neffy in the opposite nostril (R/L) were also numerically higher than repeat doses of 0.3 mg injection for approximately 30 minutes, which is longer than the time period when clinical response would be expected to be observed after dosing epinephrine (i.e., within 10 minutes).
The PK profile of repeat doses of neffy during normal nasal conditions was shown in prior studies to be highly similar to repeat doses of EpiPen, which serves as the upper bracket for exposures established to be safe. In this study, the PK profile of repeat doses of neffy in the same nostril (R/R) during nasal allergen challenge was highly similar to repeat doses of neffy during normal nasal conditions, and therefore also in the range of exposures established to be safe.
Other ARS Pharma clinical studies have evaluated neffy PK/PD in people with the same TNSS and congestion severity scores as the experimental NAC studies, but with nasal symptoms due to real-world conditions such as upper respiratory tract infections or acute allergic rhinitis from natural causes. These other studies showed no meaningful difference on PK/PD compared to dosing neffy under normal nasal conditions.
Repeat doses of neffy were considered safe and well-tolerated. All adverse events were mild and continue to be consistent with those observed in prior studies of neffy across more than 700 subjects. There were no serious adverse events.
Regulatory Status of neffy
Additional discussions with FDA subsequent to its issuance of the CRL confirmed that the new data are anticipated to be informative to labeling of administering a second dose of neffy. In May 2023, the FDA Advisory Committee (PADAC), determined a favorable benefit-risk profile of neffy (16:6 for adults and 17:5 for children). No PADAC member requested a repeat dose study during allergen-induced allergic rhinitis. In addition, multiple PADAC members highlighted the favorable profile of neffy in our single dose NAC study.
ARS Pharma intends to file its response to the FDA’s CRL for neffy early in the second quarter of 2024, with an expected PDUFA action date six months from filing, followed by potential U.S. launch of neffy in the second half of 2024. ARS Pharma also intends to submit this repeat dose study data to the European Medicines Agency (EMA) to inform its Committee for Medicinal Products for Human Use (CHMP) Opinion decision expected by mid-2024.
About Type I Allergic Reactions including Anaphylaxis
Type I severe allergic reactions are serious and potentially life-threatening events that can occur within minutes of exposure to an allergen and require immediate treatment with epinephrine, the only FDA-approved medication for these reactions. While epinephrine autoinjectors have been shown to be highly effective, there are well published limitations that result in many patients and caregivers delaying or not administering treatment in an emergency situation. These limitations include fear of the needle, lack of portability, needle-related safety concerns, lack of reliability, and complexity of the devices. There are approximately 40 million people in the United States who experience Type I severe allergic reactions. Of those, only 3.3 million currently have an active epinephrine autoinjector prescription, and of those, only half consistently carry their prescribed autoinjector. Even if patients or caregivers carry an autoinjector, more than half either delay or do not administer the device when needed in an emergency.
About ARS Pharmaceuticals, Inc.
ARS Pharma is a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect themselves from severe allergic reactions that could lead to anaphylaxis. The Company is developing neffy® (previously referred to as ARS-1), an intranasal epinephrine product in clinical development for patients and their caregivers with Type I allergic reactions including food, medications and insect bites that could lead to life-threatening anaphylaxis. For more information, visit www.ars-pharma.com.
Forward-Looking Statements
Statements in this press release that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to ARS Pharma’s belief that the results of the repeat dosing study will support the filing of the NDA; the timing for the planned NDA filing; the estimated review time of the NDA; the potential approval of neffy; the timing for the potential U.S. launch of neffy, if approved; the timing for an expected review decision from CHMP; ; and other statements that are not historical fact. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “anticipate,” “believe,” “plans,” “expects,” “intend,” “will,” “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon ARS Pharma’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, FDA did not provide guidance on a prespecified set of endpoints for the repeat dosing study; FDA may not complete its review of the planned NDA within the anticipated six-month timeframe; the results of the repeat dosing study may not support the approval of neffy; potential safety and other complications from neffy; the labelling for neffy, if approved; the scope, progress and expansion of developing and commercializing neffy; the size and growth of the market therefor and the rate and degree of market acceptance thereof vis-à-vis intramuscular injectable products; ARS Pharma’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in ARS Pharma’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, filed with the Securities and Exchange Commission on November 9, 2023. This document can also be accessed on ARS Pharma’s web page at ir.ars-pharma.com by clicking on the link “Financials & Filings.”
The forward-looking statements included in this press release are made only as of the date hereof. ARS Pharma assumes no obligation and does not intend to update these forward-looking statements, except as required by law.
ARS Media Contact:
Laura O’Neill
Laura.oneill@finnpartners.com
ARS Investor Contact:
Justin Chakma
ARS Pharmaceuticals
justinc@ars-pharma.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/ac97c7b2-3ccd-42e0-b05f-a3fb647cc475
https://www.globenewswire.com/NewsRoom/AttachmentNg/6f47c739-a27a-4574-a1e0-48744ba9cd65
https://www.globenewswire.com/NewsRoom/AttachmentNg/4869ee96-5114-4356-b619-29425c5d23fd















