 (NewsUSA)
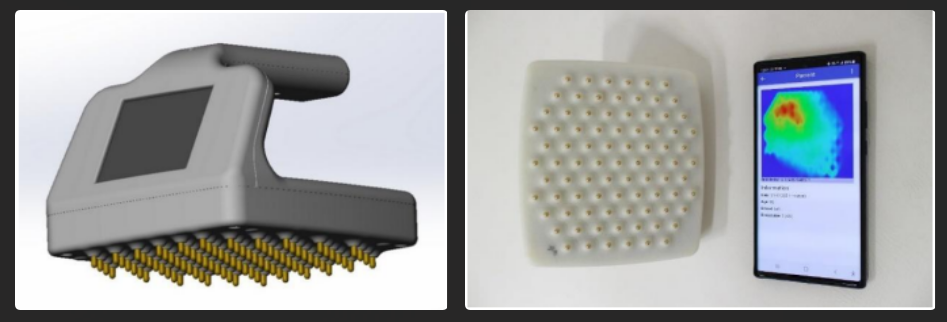
(NewsUSA) - The ScanEase OneSense device is a screening tool for diagnosing breast cancer. The device facilitates an examination that is accessible to patients of any age and body type, allowing for self-examination due to its user-friendly design in the privacy of their home. The results help assess the risk of new tissue formations in the breast. The higher the risk factor on the BI-RADS scale, the greater the likelihood of malignant tissue degeneration. In light of this, the OneSense scanning device is recommended for the primary diagnosis of breast tumors in outpatient settings and for at-home self-examinations. The scan results are visible through an app on your smartphone, which will advise whether you should consult with a physician.
- The ScanEase OneSense device is a screening tool for diagnosing breast cancer. The device facilitates an examination that is accessible to patients of any age and body type, allowing for self-examination due to its user-friendly design in the privacy of their home. The results help assess the risk of new tissue formations in the breast. The higher the risk factor on the BI-RADS scale, the greater the likelihood of malignant tissue degeneration. In light of this, the OneSense scanning device is recommended for the primary diagnosis of breast tumors in outpatient settings and for at-home self-examinations. The scan results are visible through an app on your smartphone, which will advise whether you should consult with a physician.
Measurement method:
The device measures the distribution of conductivity between the electrodes on the surface of the breast, which is influenced by the distribution of blood flow in the breast tissue. The device utilizes the well-known principle of bioimpedance, measuring the difference in electrical conductivity between healthy and cancerous tissues, as tumor growth significantly increases blood flow. This allows the device to demonstrate a high specificity in breast cancer diagnostics. The results obtained from using the device will enable physicians to enhance the diagnostic quality of fibroepithelial and non-epithelial breast formations, as diagnostic errors occur in 20% to 60% of cases during routine patient examinations. The widespread use of ScanEase scanning technology will reduce the reliance on X-ray mammography and MRI examinations as routine methods for assessing breast health, thereby decreasing radiation exposure.
A unique software with a proprietary AI-based algorithm has been developed, enabling users to determine the risk factor for the presence of neoplasms in breast tissue immediately after screening, with an accuracy of up to 85%. The bioimpedance method identifies areas of increased electrical conductivity in a woman's mammary gland, that are formed due to increased blood flow, which is characteristic of tumor development.
The interpretation of the obtained data is performed by an AI-based system using the international BI-RADS scale, which indicates a complex 'risk factor' parameter on a specialized point scale. This approach standardizes and digitizes the data description using original algorithms, allowing for an assessment of the patient's risk according to the BI-RADS scale. The method makes it possible to reliably divide patients into 3 groups of threats based on the magnitude of the risk factor: low, medium and high risk.
The user of the scanning device can immediately view the results through an app on their smartphone. If a medium or high-risk result is indicated, the person should promptly contact their physician for further diagnosis.
In early 2025, the device will enter FDA clinical trials on a fast-track basis for a non-invasive medical trial. Upon final FDA approval, the device will be available for purchase online, with a projected cost of $350. The device can be shared among friends and family, reducing the cost per person using it. View the Company’s website at www.scanease.co or send an email to info@scanease.co to request more detailed information.













