Cutera, Inc. (Nasdaq: CUTR) ("Cutera" or the "Company"), a leading provider of aesthetic and dermatology solutions, today announced that the 12-month clinical data related to AviClear, the first and only FDA-cleared energy-based device for the treatment of mild, moderate, and severe acne, was presented at the Annual Fall Clinical Dermatology Conference, which took place October 20-23 in Las Vegas, Nevada.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221025005433/en/
(Photo: Business Wire)
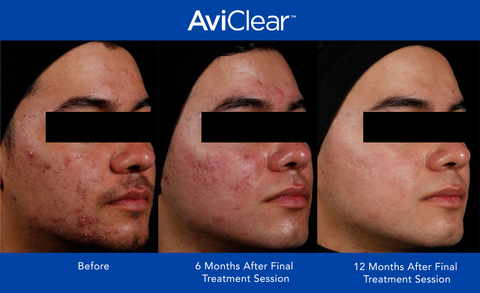
Current clinical studies show that after three 30-minute treatment sessions, 90% of patients had a visible improvement in their acne at 6 months.1 New 12-month clinical findings show this improvement increases to 92%,2 confirming the continual improvement of acne clearance and skin quality over time. Studies also demonstrate that three-fourths of patients showed a 2+ IGA score improvement and two-thirds of patients were assessed as clear or almost-clear 12 months after their final treatment session.3
Newly published articles in the Lasers in Medical Science and the Journal of Cosmetic Dermatology exemplify the proven safety and efficacy of AviClear’s method of action and the validation of Cutera’s research methods for the clinical data assessments.
Oral Presentations
Lasers and Light: Clinical and Aesthetics
David J. Goldberg, MD, JD
Therapeutic Hotline: Acne, Rosacea, AK’s, Eczema, and Others
Gary Goldenberg, MD
What’s New and Hot In Acne and Rosacea
Valerie Callender, MD, FAAD
Recent Publications
A novel 1726‑nm laser system for safe and effective treatment of acne vulgaris
Matteo Giuseppe Scopelliti, Senior Biomedical Engineer, Cutera, Amogh Kothare, VP of Clinical and Regulatory Affairs, Cutera, and Michael Karavitis, Ph. D, Executive Vice President, Chief Technology Officer, Cutera
Picture-based acne lesion counts: A validation study to assess accuracy and reliability of acne lesion counts via photography
Michael H. Gold MD, Ashish Bhatia MD, Arshdeep Kaur MS, Cutera, Margot Doucette, MS, and Amogh Kothare, VP of Clinical and Regulatory Affairs, Cutera
“We are delighted the research supports AviClear’s long-term durability. Many of my patients are thrilled with their results, and with this data , we can visibly display the longstanding effectiveness of the treatment outcomes. This is especially impactful for acne sufferers who are seeking an alternative to isotretinoin; this data proves that AviClear can be that solution for them,” said David J. Goldberg, MD, JD, board-certified dermatologist.
AviClear received FDA clearance in March 2022 following an extensive clinical trial. Select physicians began treating patients in April as part of Cutera’s limited commercial release. Interested providers and patients are encouraged to visit www.aviclear.com to sign up for updates and product alerts.
About Cutera, Inc.
Brisbane, California-based Cutera is a leading provider of aesthetic and dermatology solutions for practitioners worldwide. Since 1998, Cutera has been developing innovative, easy-to-use products that harness the power of science and nature to enable medical practitioners to offer safe and effective treatments to their patients. For more information, call +1 415-657-5500 or 1-888-4CUTERA or visit www.cutera.com.
Disclaimer for ACCME Compliance
The Annual Fall Clinical Dermatology Conference has the ultimate responsibility for the planning, development and content of continuing education programs and presentations, including those highlighted above. Cutera did not direct content or influence the planning or implementation of the Annual Fall Clinical Dermatology Conference. The opinions expressed by speakers and participants during these activities belong to those individuals.
1,2,3 Data on file. FDA clearance study. Cutera, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221025005433/en/
Contacts
Media: EvolveMKD - Cutera@EvolveMKD.com
Investor Relations: Greg Barker, VP of FP&A – IR@cutera.com













