Highlights AIM’s Significant Momentum and Ongoing Achievement of Clinical and Regulatory Milestones
Urges Shareholders to Protect AIM’s Progress by Voting for the Company’s Board of Directors and Discarding Any Proxy Materials from the Activist Group
Launches www.SafeguardAIM.com
AIM ImmunoTech Inc. (NYSE American: AIM) (“AIM” or the “Company”) today announced that the Company has filed its Definitive Proxy Statement in connection with AIM’s upcoming 2023 Annual Meeting of Stockholders (the “2023 Annual Meeting”), scheduled for December 1, 2023. The Company also sent a letter to shareholders in connection with the Annual Meeting.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231105960127/en/
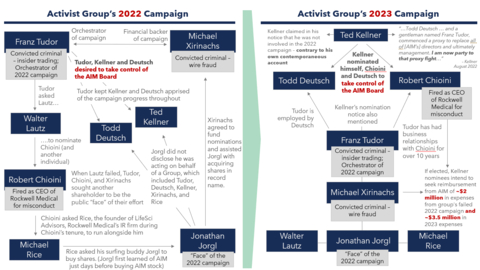
Figure 1 (Graphic: AIM ImmunoTech Inc.)
Additionally, AIM has launched www.SafeguardAIM.com to keep shareholders up to date on key developments.
The full text of the letter to shareholders follows:
November 6, 2023
Dear Fellow Shareholders:
Thank you for your investment in AIM ImmunoTech (“AIM” or the “Company”). We remain deeply committed to our mission of serving patients and delivering value for you, our shareholders. We are excited about the opportunities ahead as we continue to develop life-saving therapies – including oncology treatments for large potential markets with lethal unmet medical needs.
Last year, we told you that we were building substantial positive momentum. This remains the case, with numerous milestones achieved over the past 12 months and more expected through the rest of 2023. To protect this progress and allow it to continue, we have executed a multi-year turnaround of the business ensuring that the Company’s operational execution is fully supported by a strong cash position.
Unfortunately, as happened last year, a group of activist investors (the “Activist Group”) is trying to take over control of the AIM Board of Directors (the “Board”) to further their own self-serving motives. If successful, the Activist Group would be able to disrupt the Company’s progress and put the value of your investment in danger.
The Activist Group comprises effectively the same individuals who surfaced in 2022. As you might recall, the Board determined that the nomination notice delivered by a member of the Activist Group in connection to the 2022 Annual Meeting of Stockholders (the “2022 Annual Meeting”) was not valid because it contained numerous deficiencies and false and misleading statements in violation of the Company’s Bylaws (the “Bylaws”). Furthermore, we discovered the Activist Group contained several individuals with concerning backgrounds – including two convicted criminals: Franz N. Tudor, who was convicted of insider trading, and Michael J. Xirinachs, who pled guilty to wire fraud involving fraudulent securities trading and misuse of funds. Tudor and Xirinachs orchestrated the 2022 nominations and Xirinachs – who owns no AIM stock – agreed to fund them, along with the Activist Group’s repeat nominee Robert L. Chioini, who also owns no AIM stock. Tudor, Xirinachs and Chioini worked together for many years at Rockwell Medical prior to Chioini being terminated as CEO in 2018. A member of the Activist Group subsequently brought litigation in Delaware, but the Court agreed with the Board, noting that the Activist Group’s 2022 nomination notice “was – at best – misleading.”
This year, the Board has once again found that the Activist Group’s nomination notice (the “Notice”) – submitted by Ted D. Kellner – seeking to put forth three individuals for election as directors to our four-person Board, is invalid because it once again omits and misleads as to key information our Bylaws required Kellner to disclose regarding, among other things, those who have been involved in the deceptive campaign to acquire control of AIM. In our view, this is an attempt to mislead AIM shareholders. Numerous individuals referenced in the Notice were part of the unsuccessful campaign to take control of the Board in 2022, including the two criminals who surfaced last year. We are currently involved in litigation with the Activist Group around this issue. Unless the Court rules otherwise, the Company will not recognize the nominations and any proxies submitted or votes cast for the election of the individuals from the Activist Group will be disregarded.
We want to stress that the Bylaws exist for the protection of all shareholders. The Board intends to defend shareholders vigorously against this group of interconnected individuals, including convicted felons, who seek to disregard our Bylaws and make their own rules in an attempt to take control of AIM, without paying a control premium, and use shareholder funds for its own interests.
Your vote at the 2023 Annual Meeting of Stockholders (the “2023 Annual Meeting”) scheduled for December 1st is critical in order to safeguard AIM and maintain our promising momentum. This is why we urge you to vote for the election of your incumbent Board members – Stewart L. Appelrouth, Nancy K. Bryan, Thomas K. Equels and Dr. William M. Mitchell.
Consider the following:
AIM Is Building on Substantial Momentum to Bring New Therapies to Market
We continue to be laser-focused on our development programs and achieving upcoming clinical and regulatory milestones. Our pipeline includes treatments for a range of life-threatening cancers, debilitating immune disorders and viral conditions (including Long COVID). Our lead product, Ampligen, is an immuno-modulator with significant opportunity across multiple high-value disease areas, and we continue to explore its broad application potential through our R&D efforts.
We are able to continue developing Ampligen and stay focused on clinical execution due to the Company’s prudent financial management over the past year. We have reduced R&D and G&A expenses, and the Company’s cash position is expected to fund operations across multiple key milestones through the end of 2024. It is imperative that we protect this progress.
Recent clinical highlights include:
- September 2023: Received complete topline data report from Roswell Park Comprehensive Cancer’s Phase 1 study evaluating Ampligen (rintatolimod) as a component of a CKM regimen for the treatment of early-stage triple negative breast cancer (TNBC). The results confirmed the treatment was well tolerated, with promising clinical activity of pathologic complete response (pCR) + microinvasive residual disease (ypTmic) at 66%, comparable to pembrolizumab/neoadjuvant chemotherapy (NAC).
- August 2023: Ampligen was identified as one of two potential therapeutics possessing modest to high potential for the treatment of post-COVID conditions out of 22 identified ongoing randomized clinical trials in a recent peer-reviewed publication.
- August 2023: Received updated data from Early Access Program (EAP) at Erasmus Medical Center which bolsters previously published data indicating that treatment with Ampligen following FOLFIRINOX was associated with improved survival rates in pancreatic cancer patients compared to matched controls of patients who did not receive Ampligen.
- August 2023: Commenced and completed full enrollment in the Company’s Phase 2 study evaluating Ampligen as a potential therapeutic for people with post-COVID conditions (AMP-518).
- June 2023: Announced the publication of pre-clinical data that suggests Ampligen has the potential to act directly on tumor cells to reduce tumor cell growth in pancreatic cancer patients with sufficient tumor levels of TLR-3, indicating a potential biomarker to identify patients who may respond to Ampligen. The anti-tumor analysis was published in the peer-reviewed journal American Journal of Cancer Research in the paper “Rintatolimod: A potential treatment in patients with pancreatic cancer expressing Toll-like receptor 3.”
- June 2023: Received the required approvals from the Netherlands for Erasmus Medical Center (“Erasmus MC”) to begin a Phase 1b/2 study evaluating Ampligen in combination with AstraZeneca’s Imfinzi, an FDA approved checkpoint inhibitor in specific cancers, under the previously announced external sponsored collaborative clinical research agreement with AstraZeneca and Erasmus MC.
- June 2023: Announced the opening of an additional clinical trial site at the University of Nebraska for Phase 2 study of Ampligen for the treatment of pancreatic cancer (AMP-270).
Our Refreshed Board Has the Right Industry Experience and Backgrounds to Successfully Lead AIM Forward
Under the current Board, AIM is positioned to capitalize on its significant momentum and achieve additional clinical milestones in 2024. Your current Board members – and candidates for election this year – include:
- Stewart L. Appelrouth – Mr. Appelrouth has valuable financial and regulatory expertise as a certified public accountant with over 40 years of accounting and audit experience. His extensive experience as an accountant and provider of business and tax consulting services equips him to serve as Chairman of the Board’s Audit Committee.
- Nancy K. Bryan – Ms. Bryan possesses deep commercial, marketing, business development and corporate finance expertise from her service at major pharmaceutical companies including Merck, GlaxoSmithKline and Bayer Pharmaceuticals as well as startup biotech companies including Indevus Pharmaceuticals and NPS Pharmaceuticals. She was named a director in March 2023 as part of AIM’s effort to bring additional diverse perspectives and biotechnology commercialization experience into the boardroom.
- Thomas K. Equels, M.S., J.D. – Mr. Equels is Executive Vice Chairman, Chief Executive Officer and President and has over 25 years of experience as a practicing attorney specializing in complex business litigation. He also has extensive experience in clinical trial design and development, creating intellectual property concepts and in financing drug development.
- Dr. William M. Mitchell – Dr. Mitchell serves as Chairman of the Board and has extensive medical industry experience, including as a Professor of Pathology at Vanderbilt University School of Medicine, a board-certified physician and a former member of the board of directors of Chronix Biomedical, a company involved in next-generation DNA sequencing for medical diagnostics.
The Activist Group is Not Acting in the Best Interests of AIM Shareholders and Cannot be Trusted With Your Investment
We believe the Activist Group has a self-interested agenda that will put your investment in AIM at serious risk. In fact, the Activist Group appears motivated in part by its attempt to get reimbursed for the millions of dollars of expenses it has incurred in trying to take over the AIM Board.
The Activist Group has disclosed that its members “intend to seek reimbursement from the Company of all expenses” for not only their 2023 campaign and litigation but also all expenses incurred in connection with last year’s failed campaign. In total, the Activist Group intends to seek reimbursement from the Company for expenses that they estimate will exceed $5.5 million. They have also indicated that they do not intend to submit the matter of their reimbursement to a shareholder vote if their nominees are elected, meaning that they plan to force you – the AIM shareholders – to foot the bill for their millions of dollars of expenditures without giving you any say. Remarkably, over $2 million of this reimbursement would be used to repay Chioini and his long-time associate and known felon, Xirinachs – neither of whom is even an AIM shareholder – for their failed efforts to take control of the Company in 2022.
The Delaware Court of Chancery described the Activist Group as a “web of individuals” working together to nominate a slate of nominees to take control of the Board in 2022. As noted above, the Activist Group’s interconnections and similar composition this year is striking. (See Figure 1).
AIM is committed to protecting the best interests of all shareholders and positioning the Company to capitalize on important upcoming clinical milestones. We encourage you to vote on the WHITE card to safeguard AIM and support its highly qualified Board.
To learn more, shareholders are encouraged to visit: www.SafeguardAIM.com
Sincerely,
The AIM ImmunoTech Board of Directors
***
WE URGE YOU TO COMPLETE, DATE AND SIGN THE ENCLOSED WHITE PROXY CARD AND MAIL IT PROMPTLY IN THE POSTAGE-PAID ENVELOPE PROVIDED, OR VOTE BY TELEPHONE OR THE INTERNET AS INSTRUCTED ON THE WHITE PROXY CARD, WHETHER OR NOT YOU PLAN TO ATTEND THE 2023 ANNUAL MEETING.
THE BOARD RECOMMENDS A VOTE “FOR ALL” OF OUR BOARD’S NOMINEES (STEWART L. APPELROUTH, NANCY K. BRYAN, THOMAS K. EQUELS AND DR. WILLIAM M. MITCHELL) ON PROPOSAL 1 USING THE ENCLOSED WHITE PROXY CARD.
About AIM ImmunoTech Inc.
AIM ImmunoTech Inc. is an immuno-pharma company focused on the research and development of therapeutics to treat multiple types of cancers, immune disorders and viral diseases, including COVID-19. The Company’s lead product is a first-in-class investigational drug called Ampligen® (rintatolimod), a dsRNA and highly selective TLR3 agonist immuno-modulator with broad spectrum activity in clinical trials for globally important cancers, viral diseases and disorders of the immune system.
For more information, please visit aimimmuno.com and connect with the Company on Twitter, LinkedIn, and Facebook.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”). Words such as “may,” “will,” “expect,” “plan,” “anticipate,” “continue,” “believe,” “potential,” “upcoming” and other variations thereon and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. Many of these forward-looking statements involve a number of risks and uncertainties. The Company urges investors to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the U.S. Securities and Exchange Commission (the “SEC”). You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. Among other things, for those statements, the Company claims the protection of safe harbor for forward-looking statements contained in the PSLRA. The Company does not undertake to update any of these forward-looking statements to reflect events or circumstances that occur after the date hereof.
Important Information
The Company has filed a definitive proxy statement and associated WHITE proxy card with the SEC in connection with the solicitation of proxies for the Company’s 2023 Annual Meeting. Details concerning the nominees of the Company’s Board of Directors for election at the 2023 Annual Meeting are included in the proxy statement. BEFORE MAKING ANY VOTING DECISION, INVESTORS AND STOCKHOLDERS OF THE COMPANY ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH OR FURNISHED TO THE SEC, INCLUDING THE COMPANY’S PROXY STATEMENT AND ANY AMENDMENTS OR SUPPLEMENTS THERETO, WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION.
Investors and stockholders will be able to obtain a copy of the definitive proxy statement, any amendments or supplements thereto and other documents filed by the Company free of charge from the SEC’s website, www.sec.gov. Copies of these materials will also be available free of charge on AIM’s Investor Relations website at https://aimimmuno.com/sec-filings/.
Participants in the Solicitation
The Company, its directors and certain of its executive officers are participants in the solicitation of proxies from stockholders in respect of the 2023 Annual Meeting. Information regarding the names of the Company’s directors and executive officers and their respective interests in the Company by security holdings or otherwise is set forth in the Company’s Definitive Proxy Statement, filed with the SEC on November 6, 2023. To the extent holdings of such participants in the Company’s securities have changed since the amounts described in the Definitive Proxy Statement, such changes have been or will be reflected on Initial Statements of Beneficial Ownership on Form 3 or Statements of Change in Ownership on Form 4 filed with the SEC. These documents can be obtained free of charge from the sources indicated above.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231105960127/en/
Contacts
Investor Contact:
JTC Team, LLC
Jenene Thomas
833-475-8247
AIM@jtcir.com
Media Contact:
Longacre Square Partners
Joe Germani / Miller Winston
AIM@longacresquare.com













