- Independent evaluation of KP.3.1.1 and LB.1 variants shows in vitro pseudovirus neutralization potency of PEMGARDA in-line with prior variants tested
- Proprietary, ongoing SARS-CoV-2 spike analyses demonstrate consistent structural stability of the pemivibart binding site to date, which has routinely predicted sustained pemivibart in vitro neutralization activity
- Potential emerging variants, such as XEC and LP.1, encode mutations that are distal from the pemivibart binding site, and, therefore, are also not expected to meaningfully alter pemivibart activity; routine neutralization analysis will follow in ordinary course to assess potential for epistasis or allostery
WALTHAM, Mass., Sept. 23, 2024 (GLOBE NEWSWIRE) -- Invivyd, Inc. (Nasdaq: IVVD), a biopharmaceutical company devoted to delivering protection from serious viral infectious diseases, today provided detailed virology data supporting previously communicated neutralization activity results, along with a genetic and structural analysis of past and present SARS-CoV-2 variant spike proteins, including the established molecular target of pemivibart.
As part of Invivyd’s ongoing monitoring of antiviral activity, Invivyd contracts with LabCorp’s Monogram Biosciences lab to provide independent, robust virology assessments in a consistent lineal chain that allows for consideration of potential changing pemivibart potency as SARS-CoV-2 evolves. Pemivibart potency against contemporary viruses KP.3.1.1 and LB.1 remains in-line with the totality of predominant variants dating back to 2022, including isolates identified during the conduct of the CANOPY Phase 3 clinical trial from late 2023 to early 2024. Data Table 1, below, provides relevant comparative values drawn from the PEMGARDA Healthcare Providers Fact Sheet.
“Quantitative neutralization assays exhibit marked intrinsic variability. Since discovery, pemivibart has exhibited impressively stable neutralization results across a wide range of SARS-CoV-2 variants, including and through contemporary variants tested,” said Robert Allen, Ph.D., Chief Scientific Officer of Invivyd. “The values we observe thus far generally fall within the expected variability of the assay systems we employ.”
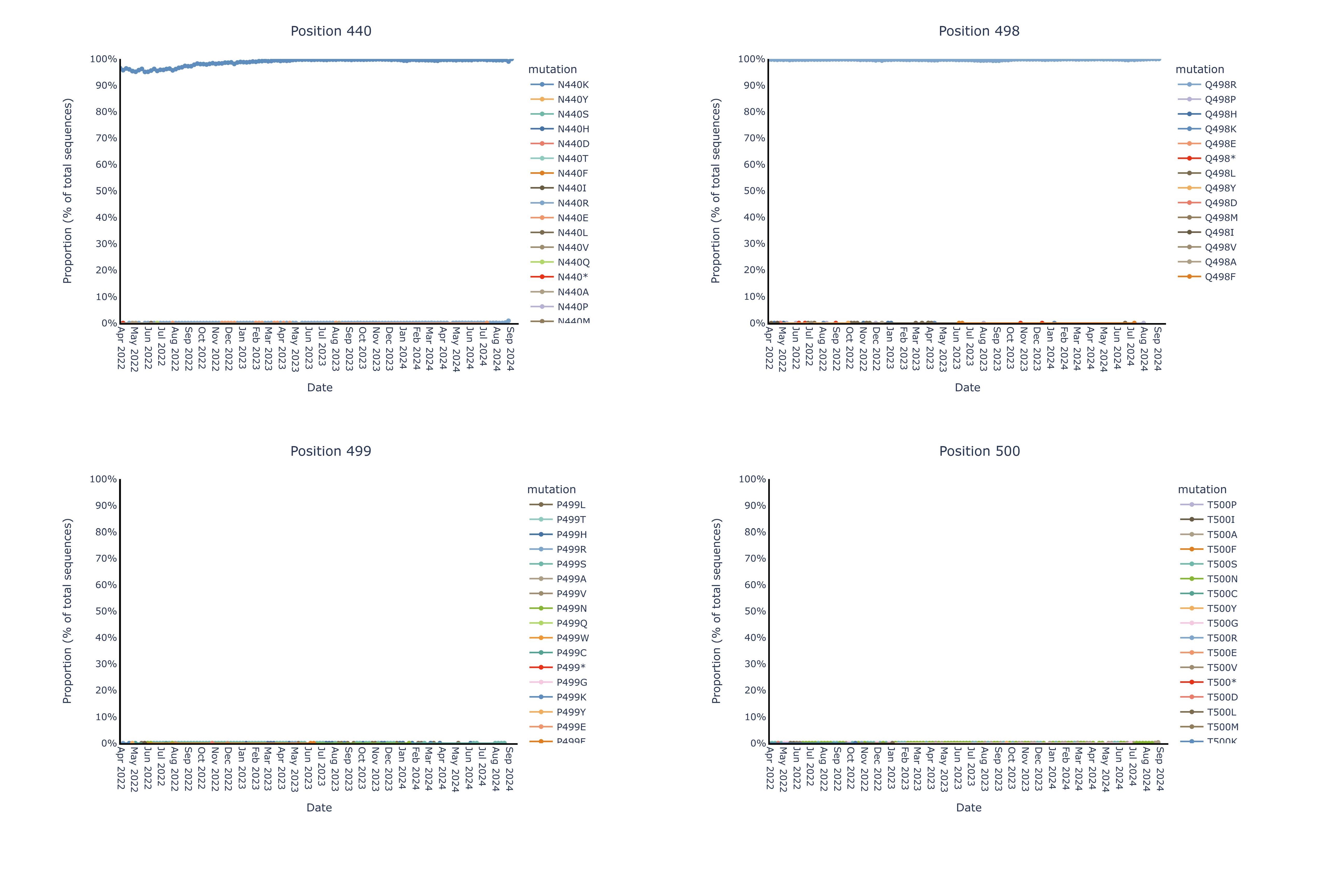
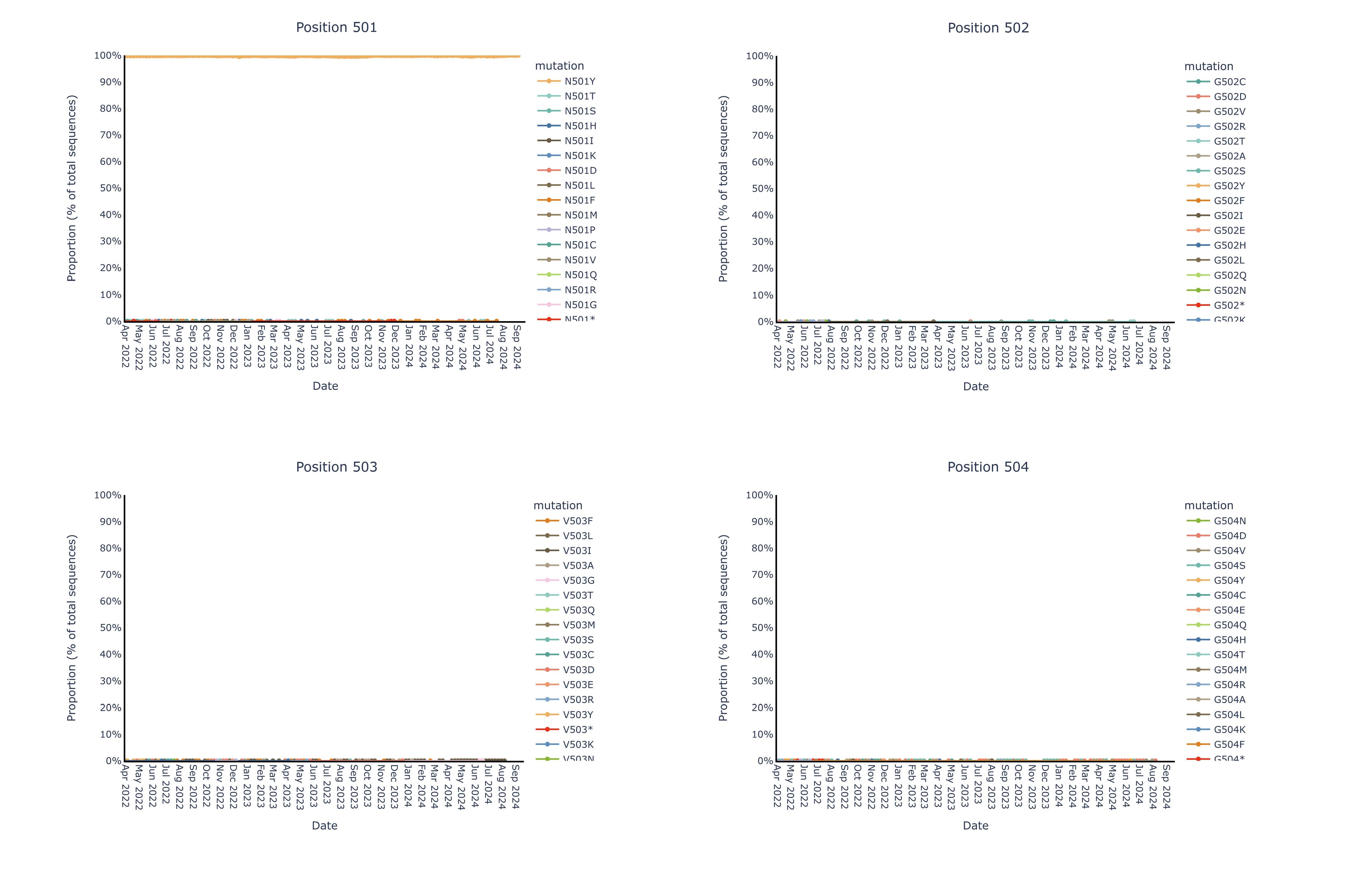
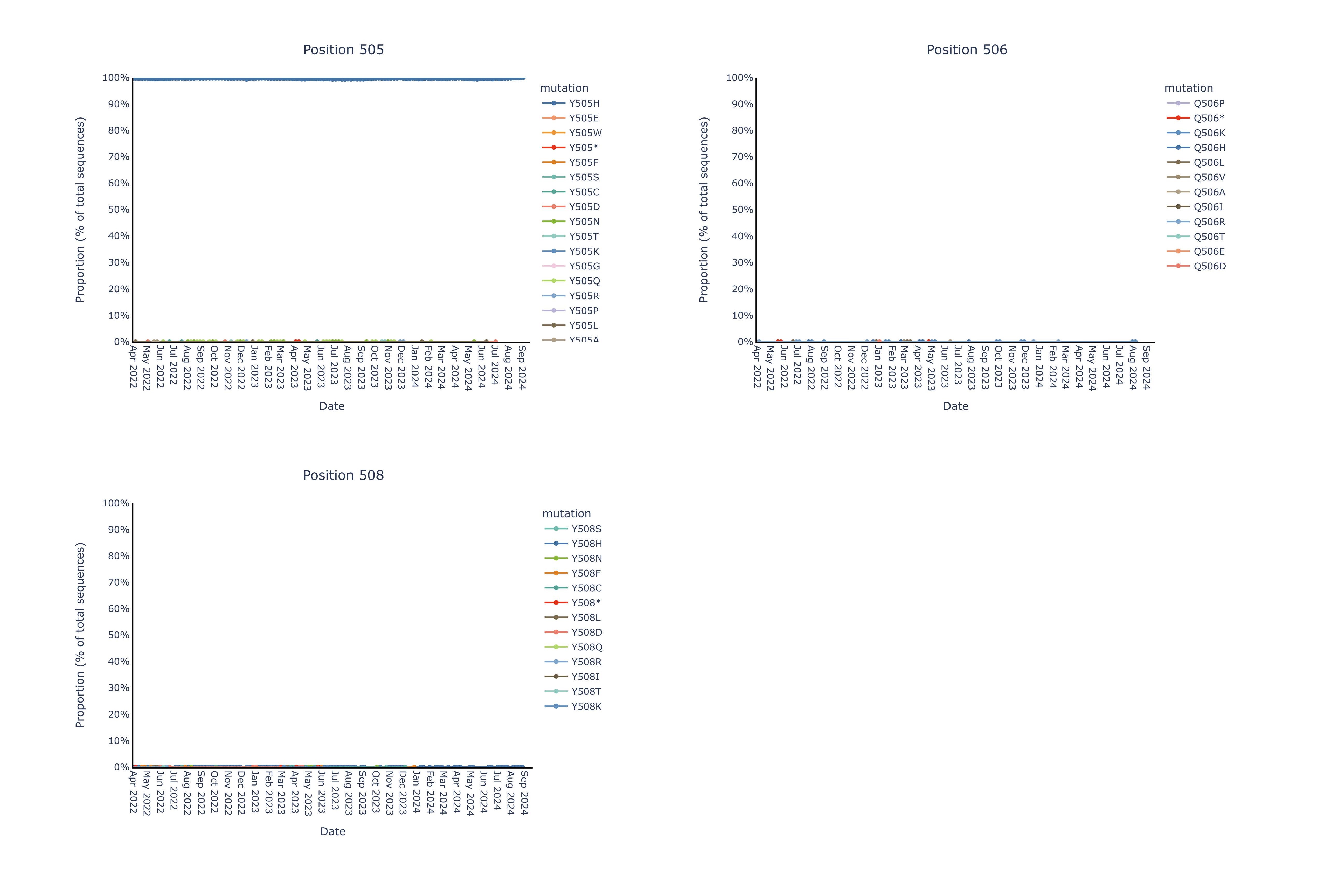
Invivyd also provided an update to ongoing structural analysis showing no meaningful mutational change in the pemivibart binding site since the Omicron shift late in 2021. These data are presented in Figure 1 below. The pemivibart binding site is defined as a region of amino acid residues on the spike protein within 5 angstroms (5 Å) of the interaction between pemivibart and spike protein in a crystal structure and consists of 19 key amino acids. Invivyd’s ongoing genetic and structural analyses of these residues provide the underlying biological rationale for the company’s ongoing expectation for neutralization activity of pemivibart in the face of constant and convergent mutation in the SARS-CoV-2 spike protein. Analysis to date of all 19 amino acids presented below shows de minimis mutational change with ongoing stability to the binding site since the Omicron shift.
"To stay ahead of the curve, it is necessary to integrate data across multiple sources, including clinical and wastewater surveillance, as well as functional genomics. Invivyd's work over the last few years has achieved exactly that,” said Kristian Andersen, Ph.D., a professor in the Department of Immunology and Microbiology at Scripps Research and member of Invivyd's scientific advisory board. "Continued surveillance of SARS-CoV-2 evolution is critically important to ensure that we can effectively counter the threat posed by the virus."
Data Table 1: Pemivibart estimated neutralization potencies1 against past and current SARS-CoV-2 viral lineages of note from LabCorp’s Monogram Biosciences lab.
Source: PEMGARDA Healthcare Providers Fact Sheet and Invivyd Data on File
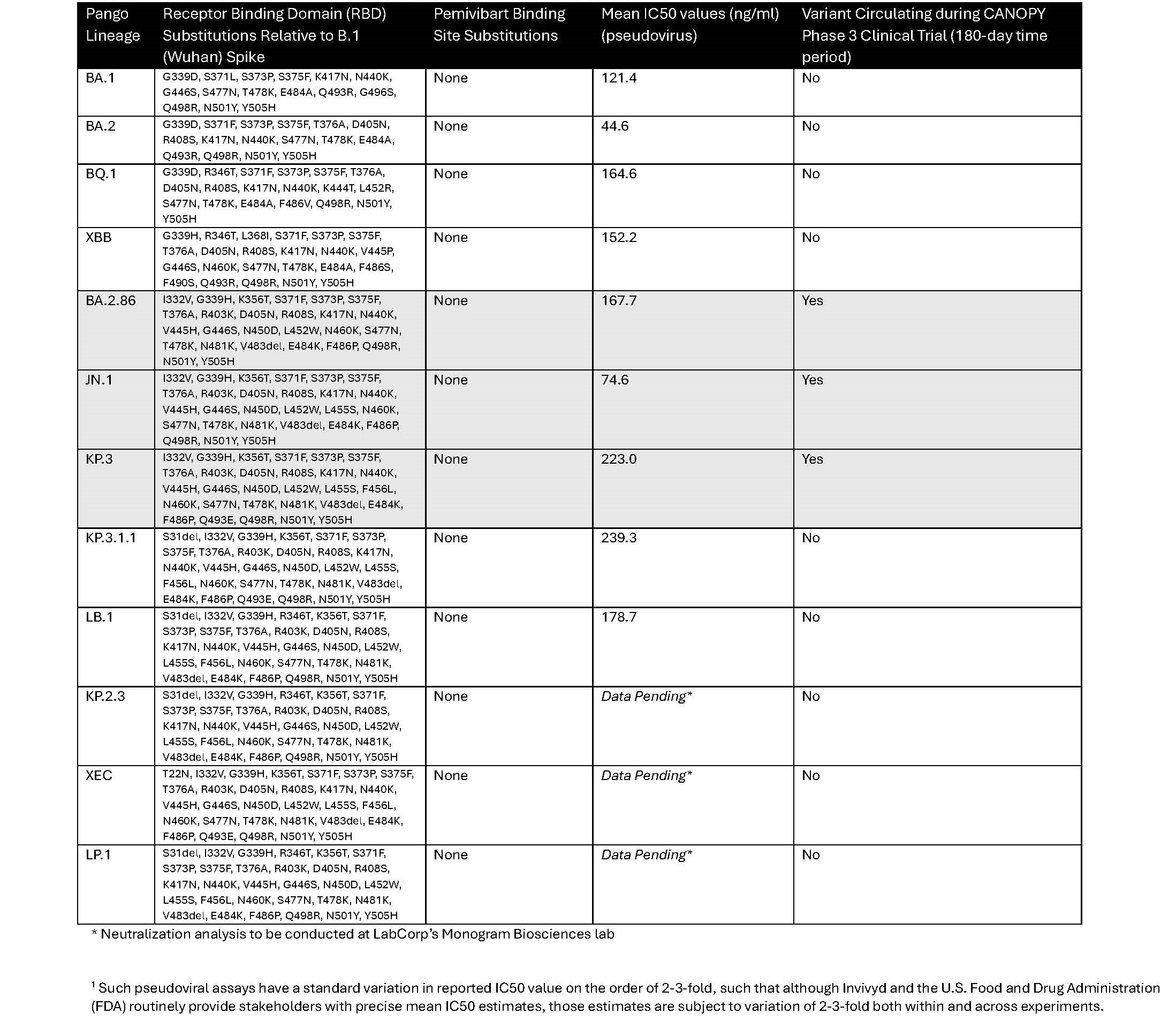
Figure 1: Updated Pemivibart Binding Site VivydTools Analysis
The following images display each amino acid residue change from original B.1 Wuhan isolate SARS-CoV-2 within the 5 Å pemivibart binding site since April 20222. Curves on the chart represent mutations appearing over time in the GISAID database of virus data, which includes more than 16 million sequences over the course of the SARS-CoV-2 pandemic. For context, presented below are sample mutations that lie within the binding sites of past COVID-19 antibodies, denoted by color and notable for clear changes in dominant amino acid over the period 2022-present.
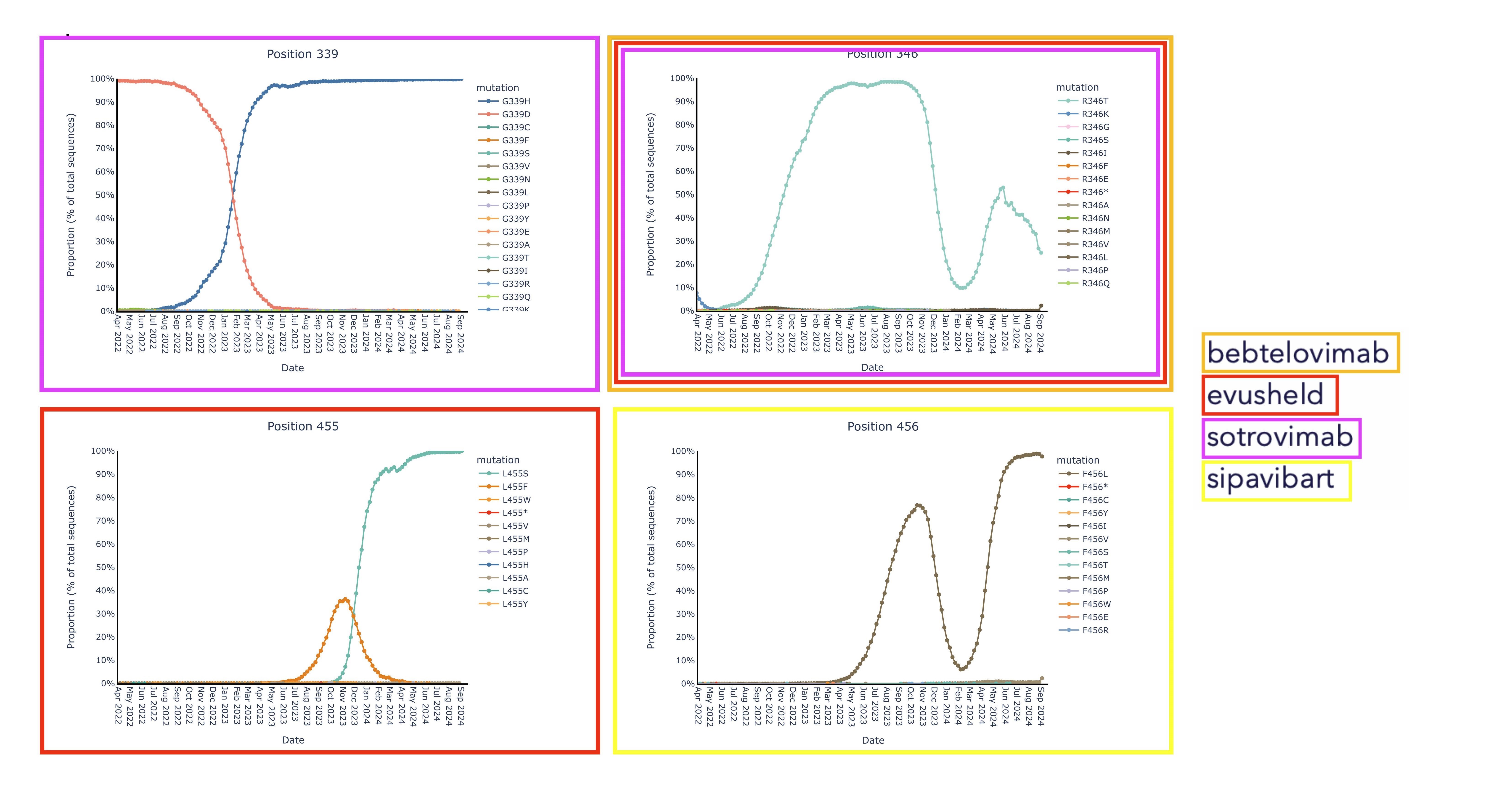
2 The x-axis (time) has been truncated for presentation purposes; all pemivibart binding site residues have demonstrated 99% or better genetic stability across all sequences deposited in GISAID since Omicron BA.1.
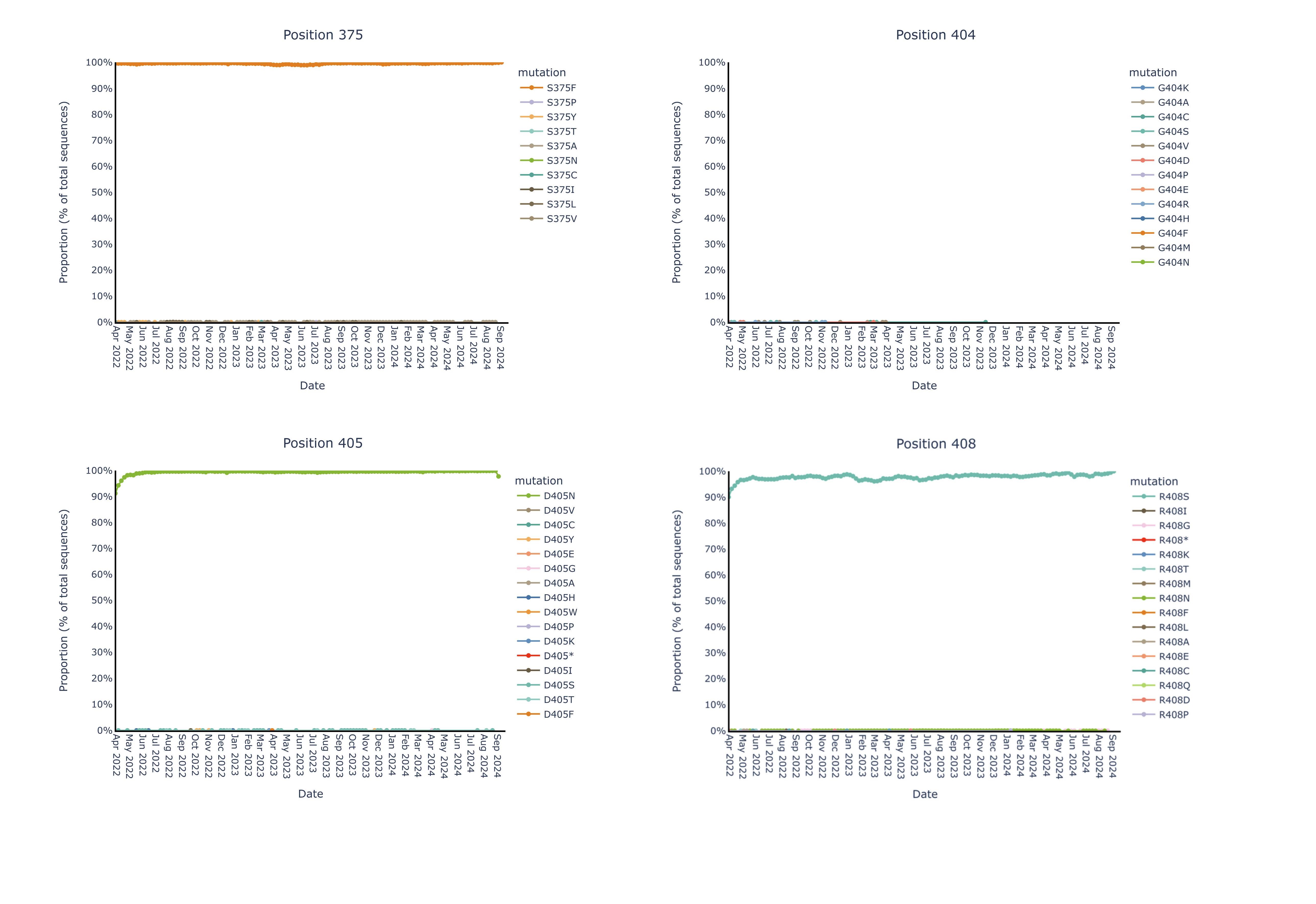
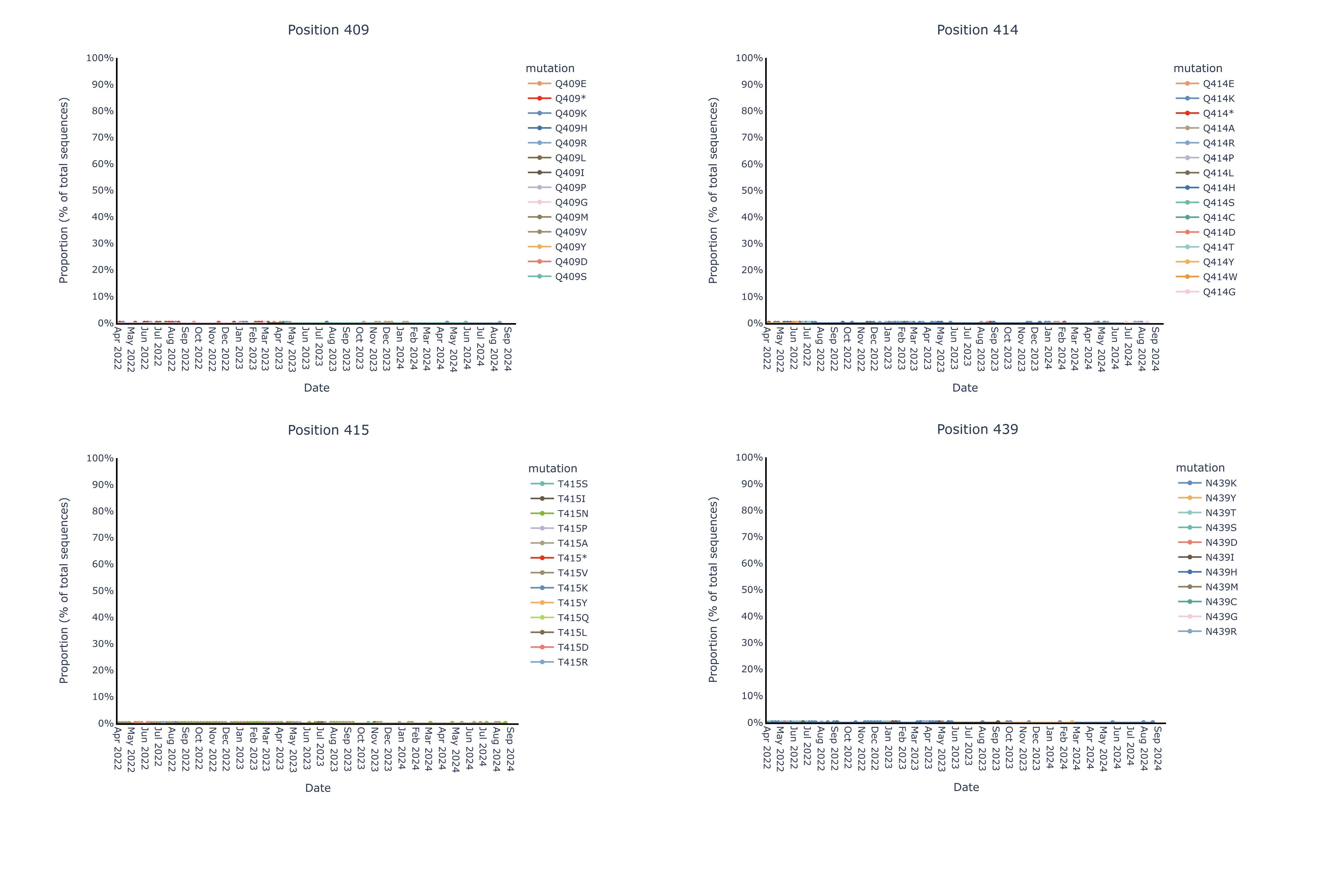
By contrast, we observe near complete (99%+) genetic and structural stability of the pemivibart binding site residues since Omicron BA.1 over the same timeframe, as displayed in the 19 charts below that have minimal to no observable change in proportion of total sequences across all COVID-19 viral sequences deposited in GISAID since 2022:
About PEMGARDA
PEMGARDA™ (pemivibart) is a half-life extended investigational monoclonal antibody (mAb). PEMGARDA was engineered from adintrevimab, Invivyd’s investigational mAb that has a robust safety data package and provided evidence of clinical efficacy in a global Phase 2/3 clinical trial for the prevention and treatment of COVID-19. PEMGARDA has demonstrated in vitro neutralizing activity against major SARS-CoV-2 variants, including JN.1. PEMGARDA targets the SARS-CoV-2 spike protein receptor binding domain (RBD), thereby inhibiting virus attachment to the human ACE2 receptor on host cells.
PEMGARDA (pemivibart) injection (4500 mg), for intravenous use is an investigational mAb that has not been approved, but has been authorized for emergency use by the FDA under an EUA for the pre-exposure prophylaxis (prevention) of COVID-19 in adults and adolescents (12 years of age and older weighing at least 40 kg) who have moderate-to-severe immune compromise due to certain medical conditions or receipt of certain immunosuppressive medications or treatments and are unlikely to mount an adequate immune response to COVID-19 vaccination. Recipients should not be currently infected with or have had a known recent exposure to an individual infected with SARS-CoV-2.
PEMGARDA is not authorized for use for treatment of COVID-19 or post-exposure prophylaxis of COVID-19. Anaphylaxis has been observed with PEMGARDA and the PEMGARDA Fact Sheet for Healthcare Providers includes a boxed warning for anaphylaxis. The most common adverse events (all grades, incidence ≥2%) observed in participants who have moderate-to-severe immune compromise treated with PEMGARDA included systemic and local infusion-related or hypersensitivity reactions, upper respiratory tract infection, viral infection, influenza-like illness, fatigue, headache, and nausea. For additional information, please see the PEMGARDA full product Fact Sheet for Healthcare Providers, including important safety information and boxed warning.
To support the EUA for PEMGARDA, an immunobridging approach was used to determine if PEMGARDA may be effective for pre-exposure prophylaxis of COVID-19. Immunobridging is based on the serum virus neutralizing titer-efficacy relationships identified with other neutralizing human mAbs against SARS-CoV-2. This includes adintrevimab, the parent mAb of pemivibart, and other mAbs that were previously authorized for EUA. There are limitations of the data supporting the benefits of PEMGARDA. Evidence of clinical efficacy for other neutralizing human mAbs against SARS-CoV-2 was based on different populations and SARS-CoV-2 variants that are no longer circulating. Further, the variability associated with cell-based EC50 value determinations, along with limitations related to pharmacokinetic data and efficacy estimates for the mAbs in prior clinical trials, impact the ability to precisely estimate protective titer ranges. Additionally, certain SARS-CoV-2 viral variants may have substantially reduced susceptibility to PEMGARDA, and PEMGARDA may not be effective at preventing COVID-19 caused by these SARS-CoV-2 viral variants.
The emergency use of PEMGARDA is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner. PEMGARDA is authorized for use only when the combined national frequency of variants with substantially reduced susceptibility to PEMGARDA is less than or equal to 90%, based on available information including variant susceptibility to PEMGARDA and national variant frequencies.
About Invivyd
Invivyd, Inc. (Nasdaq: IVVD) is a biopharmaceutical company devoted to delivering protection from serious viral infectious diseases, beginning with SARS-CoV-2. The company’s proprietary INVYMAB™ platform approach combines state-of-the-art viral surveillance and predictive modeling with advanced antibody engineering. INVYMAB is designed to facilitate the rapid, serial generation of new monoclonal antibodies (mAbs) to address evolving viral threats. In March 2024, Invivyd received emergency use authorization (EUA) from the U.S. FDA for its first mAb in a planned series of innovative antibody candidates. Visit https://invivyd.com/ to learn more.
Cautionary Note Regarding Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “anticipates,” “believes,” “could,” “expects,” “estimates,” “intends,” “potential,” “predicts,” “projects,” and “future” or similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements include statements concerning, among other things, the company’s ongoing research and clinical development activities, as well as future potential research and clinical development efforts; the company’s predictions regarding anticipated neutralization activity of PEMGARDA™ (pemivibart) as a result of virology data and analysis of SARS-CoV-2 structural biology; the company’s ongoing monitoring of antiviral activity of pemivibart as SARS-CoV-2 evolves and the company’s ability to stay ahead of the curve; the company’s expectations regarding pemivibart neutralization activity against potential emerging variants, such as XEC and LP.1, given their encoded mutations, and the company’s plans to conduct routine neutralization analysis of such variants in ordinary course; the potential benefits of continued surveillance of SARS-COV-2 evolutions; the company’s devotion to delivering protection from serious viral infectious diseases, beginning with SARS-CoV-2; the design of the company’s INVYMAB platform approach to facilitate the rapid, serial generation of new mAbs to address evolving viral threats; the potential of PEMGARDA as a mAb for pre-exposure prophylaxis (prevention) of COVID-19 in certain adults and adolescents who have moderate-to-severe immune compromise; and other statements that are not historical fact. The company may not actually achieve the plans, intentions or expectations disclosed in the company’s forward-looking statements and you should not place undue reliance on the company’s forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the company’s actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the timing, progress, and results of the company’s discovery, preclinical and clinical development activities; the risk that results of nonclinical studies or clinical trials may not be predictive of future results, and interim data are subject to further analysis; unexpected safety or efficacy data observed during preclinical studies or clinical trials; the predictability of clinical success of the company’s product candidates based on neutralizing activity in nonclinical studies; the predictability of sustained pemivibart in vitro neutralization activity based on the company’s SARS-CoV-2 spike analyses demonstrating consistent structural stability of the pemivibart binding site; potential variability in neutralizing activity of product candidates tested in different assays, such as pseudovirus assays and authentic assays; the company’s reliance on third parties with respect to virus assay creation and product candidate testing and with respect to its clinical trials; variability of results in models used to predict activity against SARS-CoV-2 variants; whether pemivibart or any other product candidate is able to demonstrate and sustain neutralizing activity against major SARS-CoV-2 variants, particularly in the face of viral evolution; how long the EUA granted by the FDA for PEMGARDA will remain in effect and whether the EUA is revoked or revised by the FDA; the company’s ability to build and maintain sales, marketing and distribution capabilities to successfully commercialize PEMGARDA; uncertainties related to the regulatory authorization or approval process, and available development and regulatory pathways for authorization or approval of the company’s product candidates; the ability to maintain a continued acceptable safety, tolerability and efficacy profile of any product candidate following regulatory authorization or approval; changes in the regulatory environment; changes in expected or existing competition; the complexities of manufacturing mAb therapies; the company’s ability to leverage its INVYMAB platform approach to facilitate the rapid, serial generation of new mAbs to address evolving viral threats; any legal proceedings or investigations relating to the company; the company’s ability to continue as a going concern; and whether the company has adequate funding to meet future operating expenses and capital expenditure requirements. Other factors that may cause the company’s actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended December 31, 2023 and the company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, each filed with the Securities and Exchange Commission (SEC), and in the company’s other filings with the SEC, and in its future reports to be filed with the SEC and available at www.sec.gov. Forward-looking statements contained in this press release are made as of this date, and Invivyd undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law.
This press release contains hyperlinks to information that is not deemed to be incorporated by reference in this press release.
Contacts:
Media Relations
(781) 208-0160
media@invivyd.com
Investor Relations
(781) 208-0160
investors@invivyd.com
Data Table 1 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e831e4e2-d67f-4c48-b598-d1b851a994d6
Figure 1 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b1919cbe-ecb6-4eb5-887f-7f1445cbbb65
Figure for Positions 375, 404, 405, 408 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b475f30a-1c50-4bfa-88cc-c077692d04ba
Figure for Positions 409, 414, 415, 439 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/18b2ce68-315b-4fb7-8b5b-a101f8fa44c5
Figure for Positions 440, 498, 499, 500 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c78ca335-31b4-4805-a042-8bdf767553dd
Figure for Positions 501, 502, 503, 504 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/cd2ed5ab-1e93-49c8-9500-318d37471c05
Figure for Positions 505, 506, 508 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ef1159f2-0b0b-4e2f-9443-9c2508f6293e















